Dr. David Huesmann

research topic
A New Polypept(o)idic Nanoparticle Platform:
From Secondary Structures, Polypept(o)ides and New Cysteine Monomers
publications
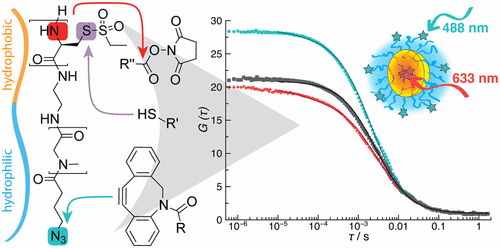
Combining Orthogonal Reactive Groups in Block Copolymers for Functional Nanoparticle Synthesis in a Single Step
Olga Schäfer, Kristina Klinker, Lydia Braun, David Huesmann, Jennifer Schultze, Kaloian Koynov, and Matthias Barz
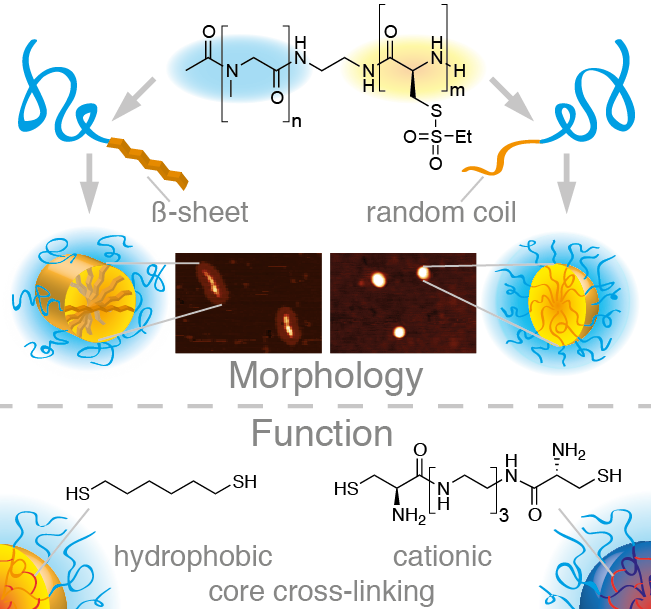
Secondary-Structure-Driven Self-Assembly of Reactive Polypept(o)ides: Controlling Size,Shape,and Function of Core Cross-Linked Nanostructures
Kristina Klinker ,Olga Schäfer, David Huesmann, Tobias Bauer, Leon Capelôa, Lydia Braun, Natascha Stergiou, Meike Schinnerer, Anjaneyulu Dirisala, Kanjiro Miyata, Kensuke Osada, Horacio Cabral, Kazunori Kataoka, Matthias Barz
Angew. Chem. Int. Ed. 2017, 56, 9608–9613.
Sekundärstrukturbildung als Triebkraft für die Selbstorganisation reaktiver Polypept(o)ide: Steuerung von Größe, Form und Funktion kernvernetzter Nanostrukturen
Kristina Klinker, Olga Schäfer, David Huesmann, Tobias Bauer, Leon Capelôa, Lydia Braun, Natascha Stergiou, Meike Schinnerer, Anjaneyulu Dirisala, Kanjiro Miyata, Kensuke Osada, Horacio Cabral, Kazunori Kataoka, Matthias Barz
Angew. Chem. 2017 , 129, 9737–9742. DOI: 10.1002/ange.201702624
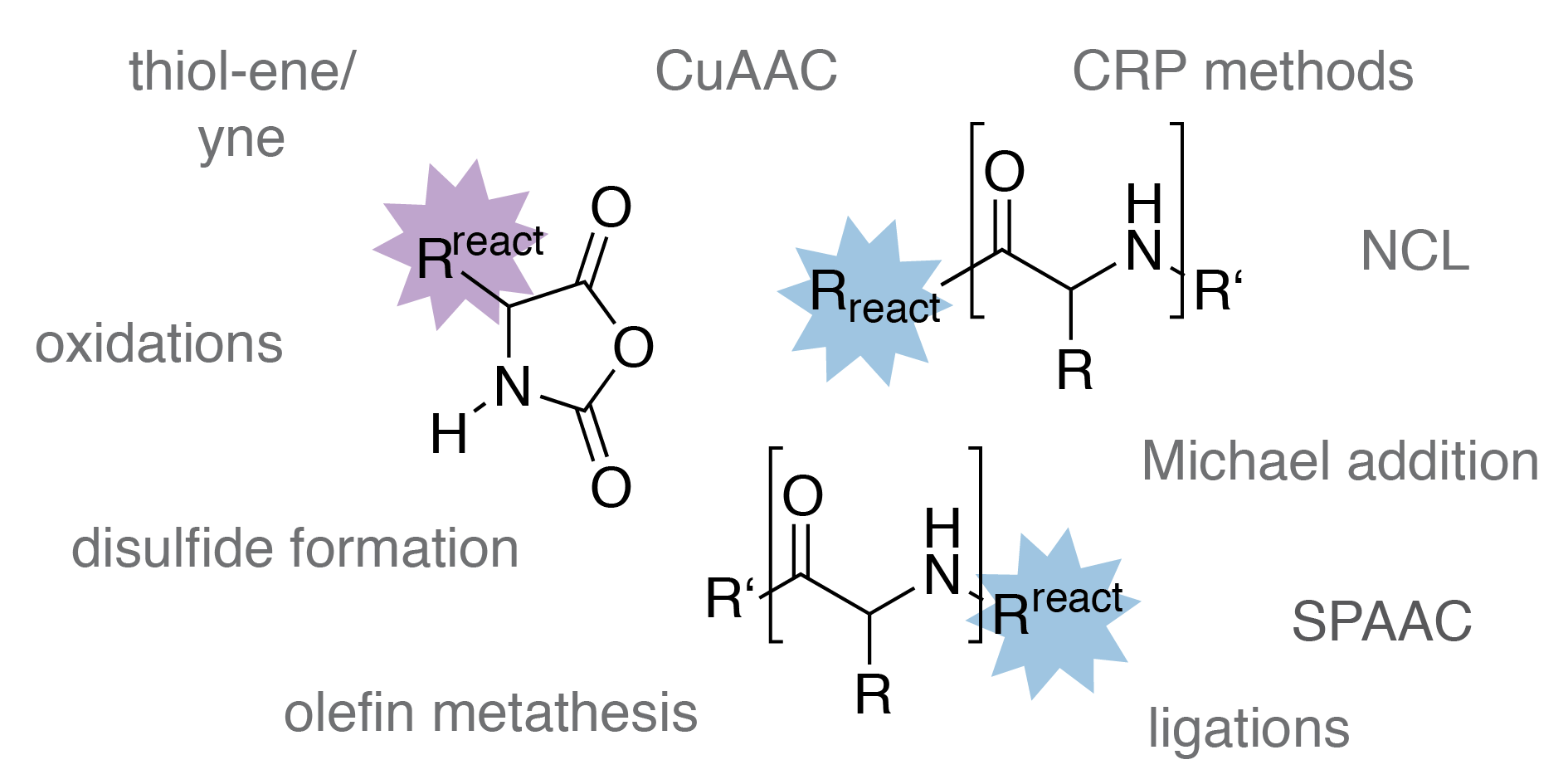
Orthogonally reactive amino acids and end groups in NCA polymerization
David Huesmann, Kristina Klinker, Matthias Barz
Polym. Chem. 2017, 8, 957–971. DOI: 10.1039/C6PY01817C
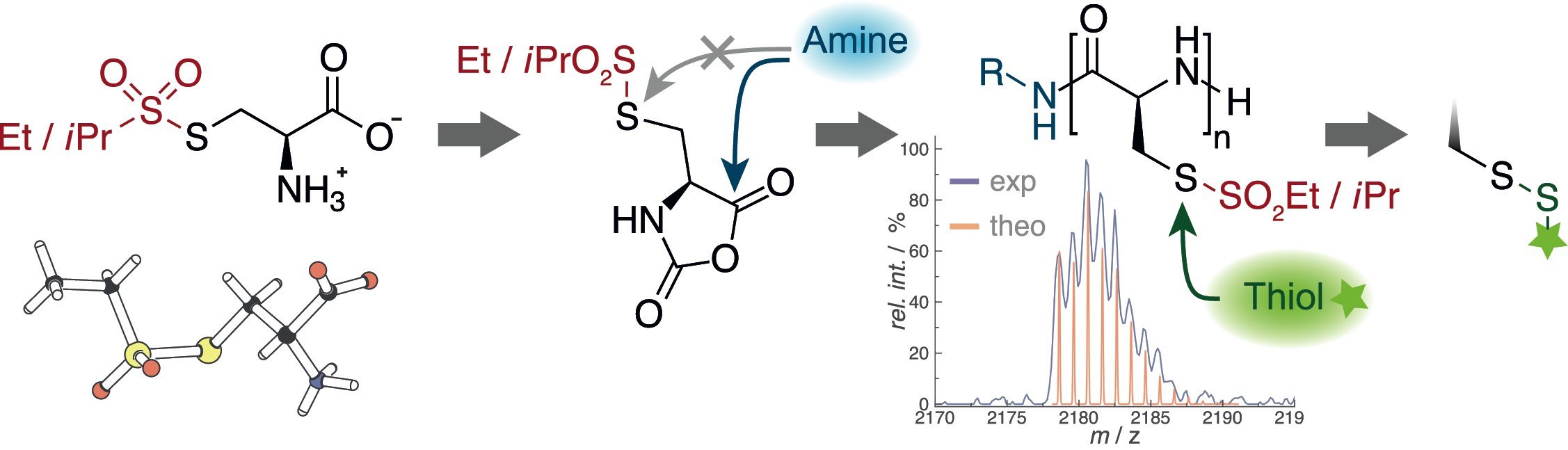
Rethinking Cysteine Protective Groups: S-Alkylsulfonyl-l-Cysteines for Chemoselective Disulfide Formation
Olga Schäfer, David Huesmann, Christian Muhl, Matthias Barz
Chem. Eur. J. 2016, 22, 18085 –18091. DOI: 10.1002/chem.201604391
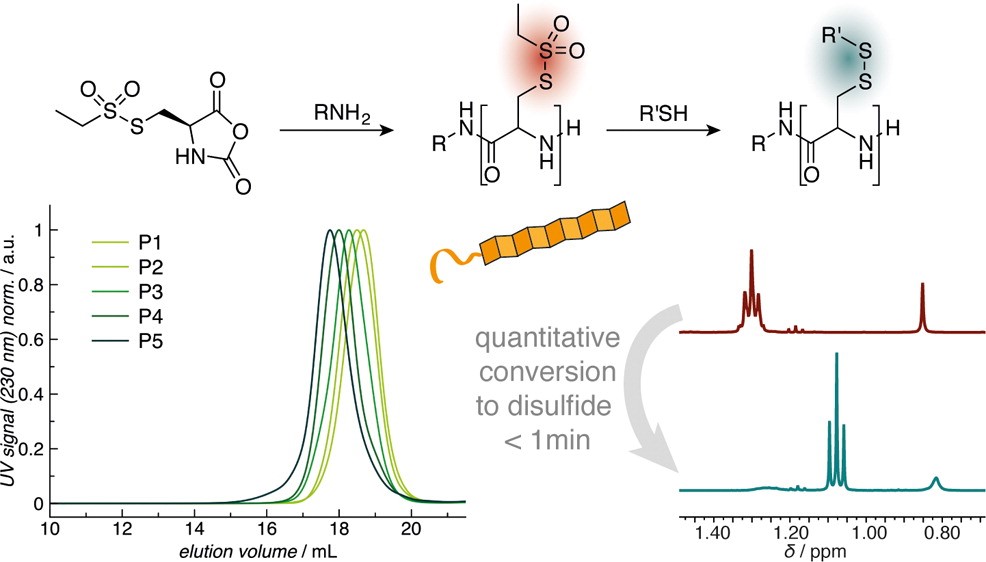
Poly(S-ethylsulfonyl-l-cysteines) for Chemoselective Disulfide Formation
Olga Schäfer, David Huesmann, Matthias Barz
Macromolecules 2016, 49, 8146–8153. DOI: 10.1021/acs.macromol.6b02064
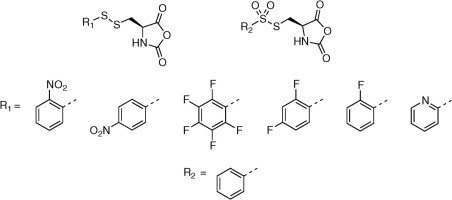
Exploring new activating groups for reactive cysteine NCAs
David Huesmann, Olga Schäfer, Lydia Braun, Kristina Klinker, Thomas Reuter, Matthias Barz
Tetrahedron Letters, 2016, 57, 1138–1142.
Bioreducible Poly-l-Lysine–Poly[HPMA] Block Copolymers Obtained by RAFT-Polymerization as Efficient Polyplex-Transfection Reagents
Kristof Tappertzhofen, Simone Beck, Evelyn Montermann, David Huesmann, Matthias Barz, Kaloian Koynov, Matthias Bros, Rudolf Zentel
Macromol. Biosci. 2016, 16, 106–120. DOI: 10.1002/mabi.201500212
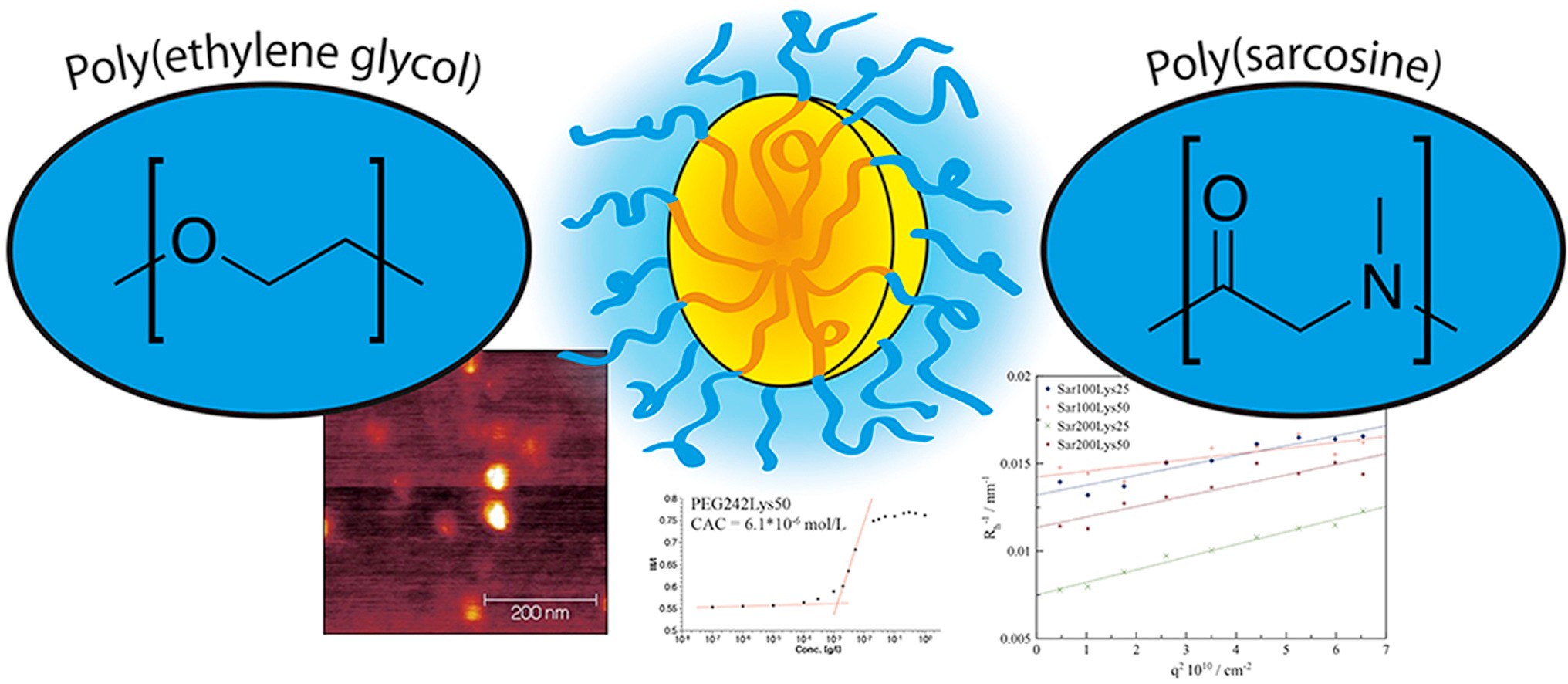
A head-to-head comparison of poly(sarcosine) and poly(ethylene glycol) in peptidic, amphiphilic block copolymers
David Huesmann, Adrian Sevenich, Benjamin Weber, Matthias Barz
Polymer 2015, 67, 240-248. DOI: 10.1016/j.polymer.2015.04.070
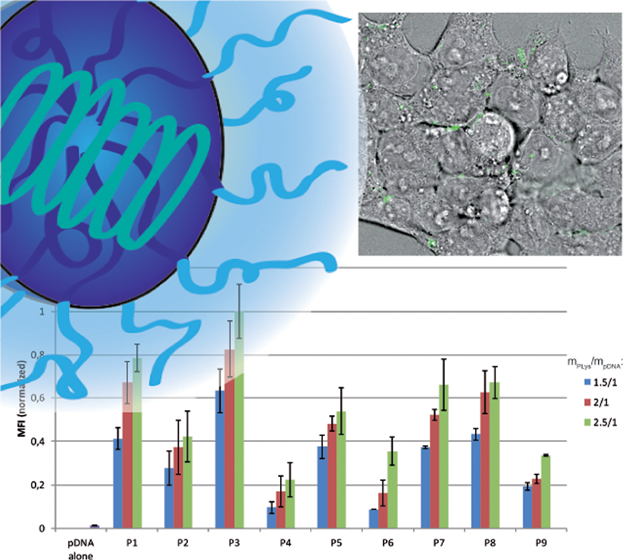
Introducing PeptoPlexes: Polylysine-block-Polysarcosine Based Polyplexes for Transfection of HEK 293T Cells
Philipp Heller, Alexander Birke, David Huesmann, Benjamin Weber, Karl Fischer, Angelika Reske-Kunz, Matthias Bros, Matthias Barz
Macromol. Biosci. 2014, 14, 1380–1395. DOI: 10.1002/mabi.201400167
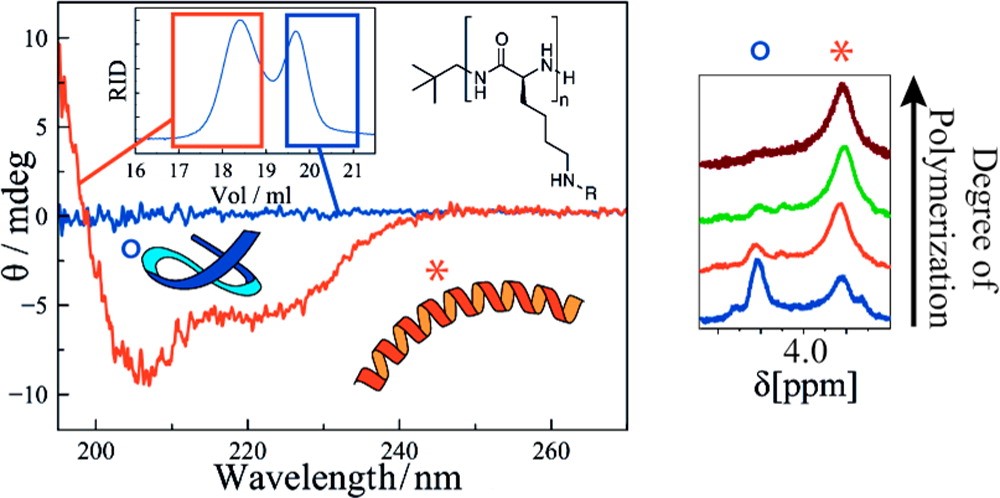
Revisiting Secondary Structures in NCA Polymerization: Influences on the Analysis of Protected Polylysines
David Huesmann, Alexander Birke, Kristina Klinker, Stephan Türk, Hans Joachim Räder, Matthias Barz
Macromolecules 2014, 47, 928−936. DOI: 10.1021/ma5000392
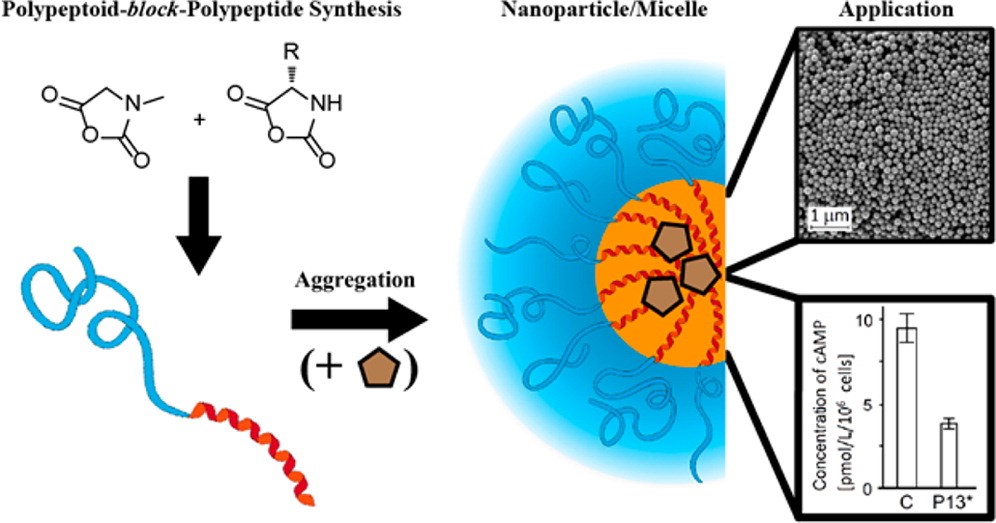
Polypeptoid-block-polypeptide Copolymers: Synthesis, Characterization, and Application of Amphiphilic Block Copolypept(o)ides in Drug Formulations and Miniemulsion Techniques
Alexander Birke, David Huesmann, Annette Kelsch, Martin Weilbächer, Jing Xie, Matthias Bros, Tobias Bopp, Christian Becker, Katharina Landfester, Matthias Barz
Biomacromolecules 2014, 15, 548−557. DOI: 10.1021/bm401542z

