Dr. Olga Schäfer

About
Olga Schäfer studied Biomedical Chemistry at the University of Toronto and JGU Mainz where she graduated in 2014. Following her pioneering work on S-ethylthiosulfonyl-L-cysteine in peptide synthesis during her diploma thesis, her PhD research focused on the development of new reactive block copolypept(o)ides and their shape controlled self-assembly into core cross-linked nanostructures for the delivery of therapeutic active components.
Contact
olga.schaefer@uni-mainz.de
Institut für Organische Chemie
Johannes Gutenberg-Universität Mainz
Duesbergweg 10-14
55128 Mainz
+49 6131 39 25468
research topic
publications
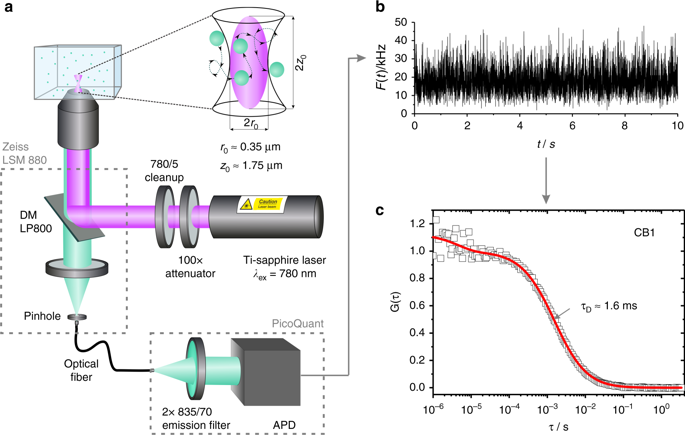
Monitoring drug nanocarriers in human blood by near-infrared fluorescence correlation spectroscopy
Inka Negwer, Andreas Best, Meike Schinnerer, Olga Schäfer, Leon Capeloa, Manfred Wagner, Manfred Schmidt, Volker Mailänder, Mark Helm, Matthias Barz, Hans-Jürgen Butt & Kaloian Koynov
Nature Communications, 9, 5306 (2018) DOI: doi.org/10.1038/s41467-018-07755-0
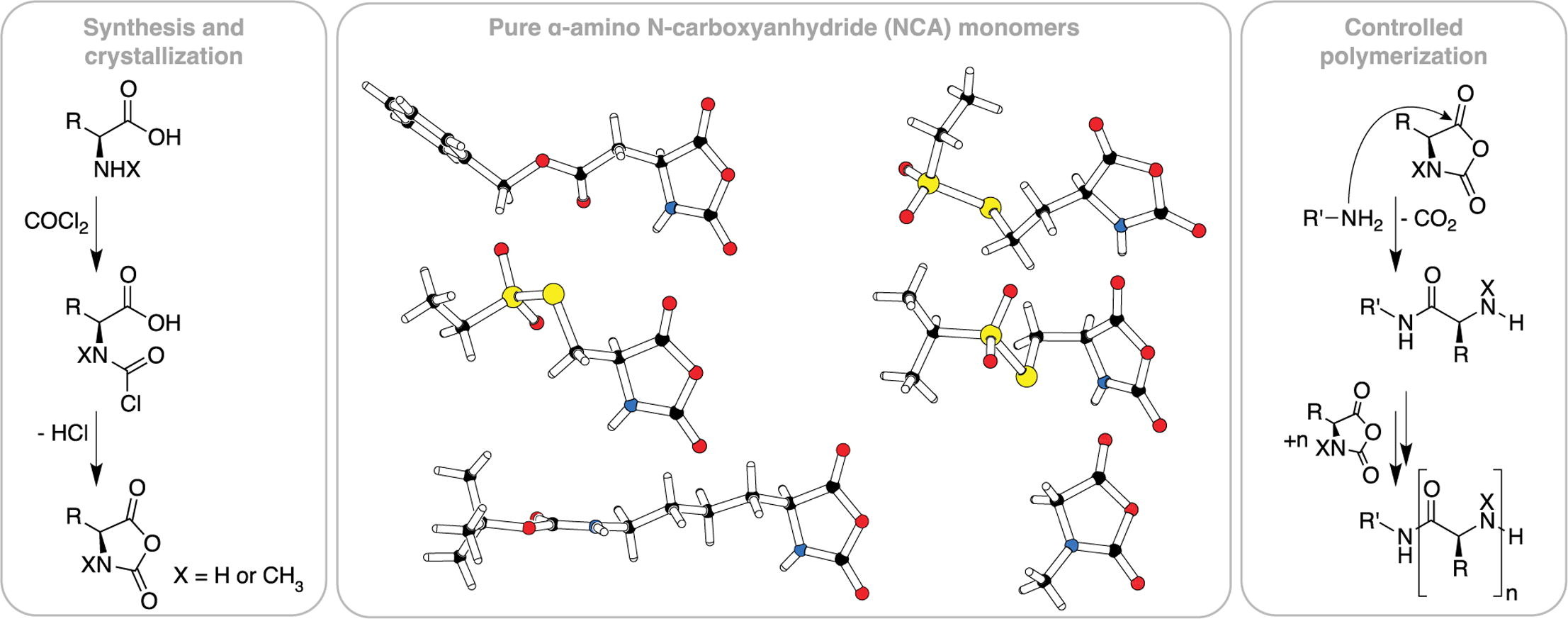
Investigation of α-amino acid N-carboxyanhydrides by X-ray diffraction for controlled ring-opening polymerization
Olga Schäfer, Dieter Schollmeyer, Alexander Birke, Regina Holm, Kerstin Johann, Christian Muhl, Christine Seidl, Benjamin Weber, Matthias Barz
Tetrahedron Letters 2019, 60, 272–275, DOI: doi.org/10.1016/j.tetlet.2018.12.028
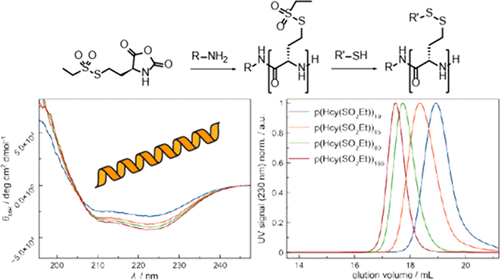
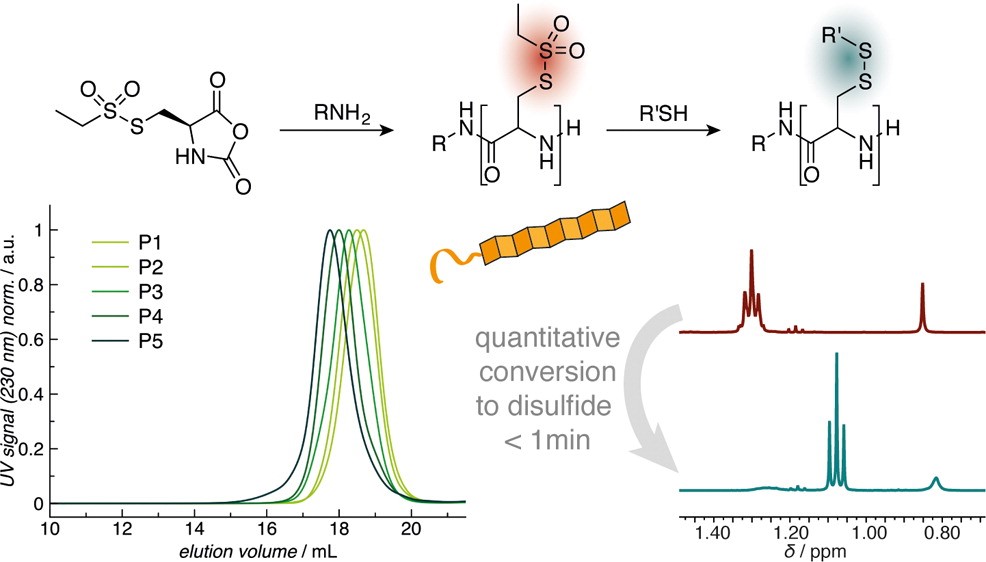
Poly(S-ethylsulfonyl-l-homocysteine): An α-Helical Polypeptide for Chemoselective Disulfide Formation
Christian Muhl, Olga Schäfer, Tobias Bauer, Hans-Joachim Räder and Matthias Barz
Macromolecules 2018, 51 (20), 8188–8196, DOI: 10.1021/acs.macromol.8b01442
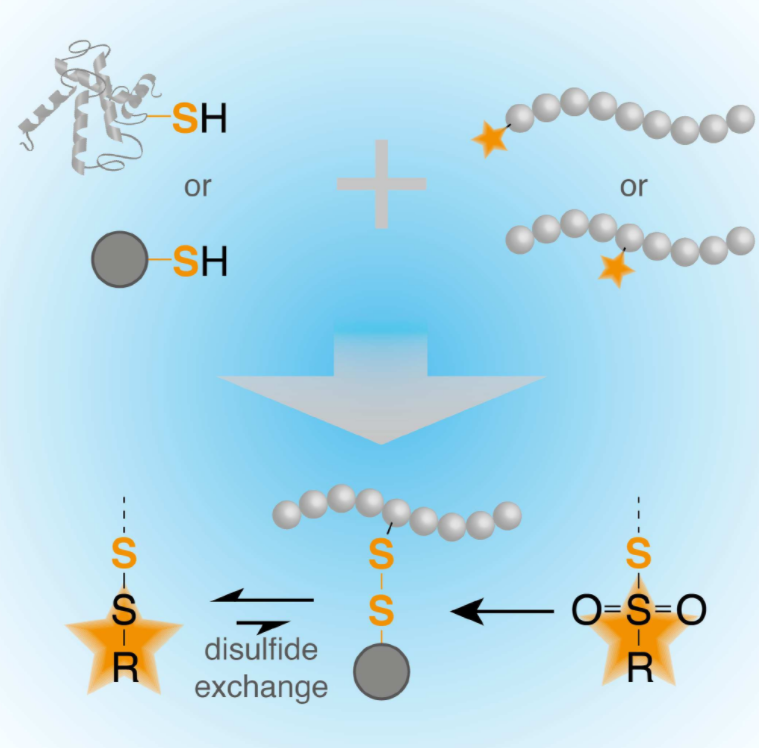
Of Thiols and Disulfides: Methods for Chemoselective Formation of Asymmetric Disulfides in Synthetic Peptides and Polymers
Olga Schäfer and Matthias Barz
Chem. Eur. J. 2018, 24, 12131–12142, DOI: 10.1002/chem.201800681
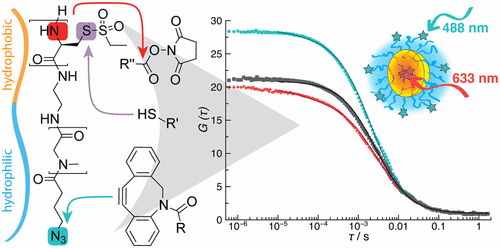
Combining Orthogonal Reactive Groups in Block Copolymers for Functional Nanoparticle Synthesis in a Single Step
Olga Schäfer, Kristina Klinker, Lydia Braun, David Huesmann, Jennifer Schultze, Kaloian Koynov, and Matthias Barz
ACS Macro Letters 2017, 6, 1140–1145, DOI: 10.1021/acsmacrolett.7b00678
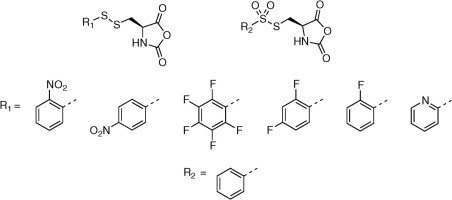
Exploring new activating groups for reactive cysteine NCAs
David Huesmann, Olga Schäfer, Lydia Braun, Kristina Klinker, Thomas Reuter, Matthias Barz
Tetrahedron Letters, 2016, 57, 1138–1142.
Rethinking Cysteine Protective Groups: S-Alkylsulfonyl-l-Cysteines for Chemoselective Disulfide Formation
Olga Schäfer, David Huesmann, Christian Muhl, Matthias Barz
Chem. Eur. J. 2016, 22, 18085 –18091. DOI: 10.1002/chem.201604391
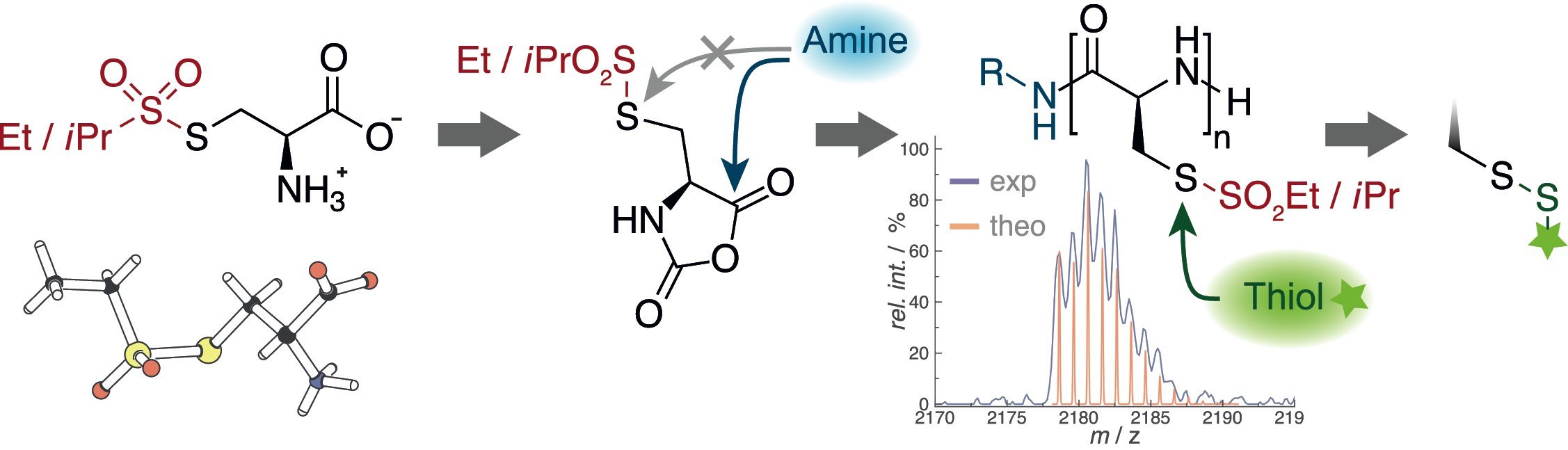
Poly(S-ethylsulfonyl-l-cysteines) for Chemoselective Disulfide Formation
Olga Schäfer, David Huesmann, Matthias Barz
Macromolecules 2016, 49, 8146–8153. DOI: 10.1021/acs.macromol.6b02064
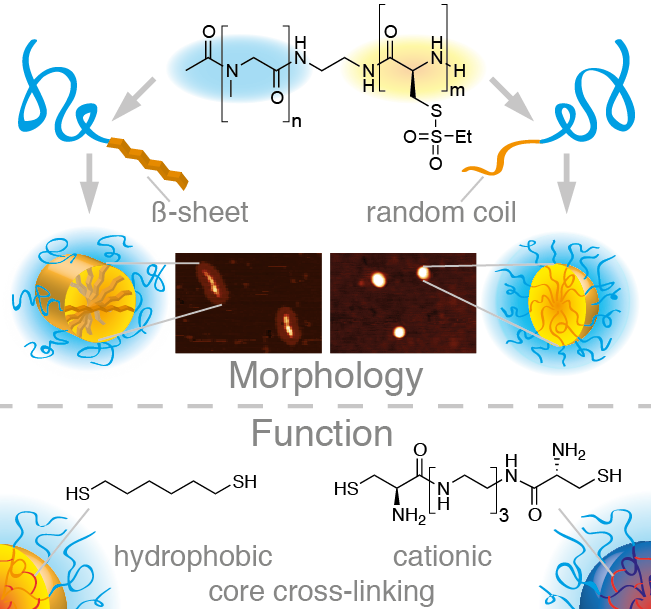
Secondary-Structure-Driven Self-Assembly of Reactive Polypept(o)ides: Controlling Size,Shape,and Function of Core Cross-Linked Nanostructures
Kristina Klinker ,Olga Schäfer, David Huesmann, Tobias Bauer, Leon Capelôa, Lydia Braun, Natascha Stergiou, Meike Schinnerer, Anjaneyulu Dirisala, Kanjiro Miyata, Kensuke Osada, Horacio Cabral, Kazunori Kataoka, Matthias Barz
Angew. Chem. Int. Ed. 2017, 56, 9608–9613.
Thiol-protected amino acid derivatives and uses thereof
Barz, D. Huesmann, O. Schäfer, T. Reuter, A. Birke, P. Heller WO2015169908A1, 2014

